MONOSEX AND STERILE FISH PRODUCED USING GENOME EDITING
The ability to produce sterile progeny from broodstock for aquaculture has significant benefits to culture productivity and environmental sustainability. We describe the development of strategies to generate, breed and mass-produce infertile fish. Our solutions rely on precise genetic modifications to create broodstock lines that can be incorporated into breeding programs. These approaches were validated in tilapia but are transferrable to multiple species of fish. We expect that adoption of these technologies will result in broad economic and environmental benefits for aquaculture.
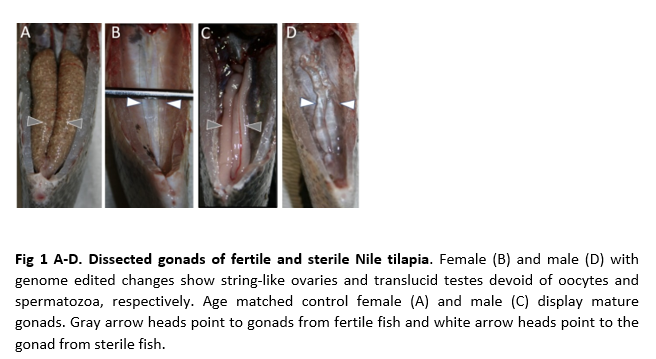
One strategy for mass producing sterile fish is designed to produce monosex, sterile populations in culture. In addition to the benefit of sterile fish, this allows the benefit of sexually dimorphic performance traits in culture. We first investigated gene mutations in two evolutionarily conserved pathways, one governing sex differentiation and the other sex competency. We created edits in genes necessary for spermiogenesis and steroid hormone synthesis causing male sterility and masculinization, respectively. Double gene edit combinations for these genes produced all-male sterile populations. Likewise, we created variants in genes whose inactivation caused females to develop atrophic ovaries arrested at a previtellogenic stage or string-like ovaries lacking oocytes. We further disrupted genes causing genetic males to sex reverse into females. Double gene edit combinations for these genes produced all-female, sterile populations.
Propagation of the double KO broodstock lines was achieved via germ cell transplantation from a juvenile donor into a germ cell free wild-type recipient embryo. In the resulting recipients, the induced edits had no effect as the genes targeted are not expressed in germ cells. With this approach, we generated fertile broodstock that successfully mass-produced sterile, monosex populations.